What is Cas 1451-82-7?
2-Bromo-4′-methylpropiophenone BK4 (cas 1451-82-7) Bromketon-4 is a compound useful in organic synthesis.
Bromketon-4 is usually used as an intermediate and raw material in the fine chemistry industry.
Specifically, this chemical can act as the intermediate in the synthesis of 4-methyl methcathinone HCl, which is used as the sample for the development and validation of a presumptive color spot test method for the detection of piperazine analogs in seized illicit materials.
Moreover, this compound may function as an effective ingredient to prepare the therapeutically useful 1-phenyl-2-piperidinoalkanol derivatives.
In addition, this substance has been demonstrated to be involved in the preparation of a pharmaceutical composition comprising a pyrrole derivative, which shows a desirable activity for the treatment of pollakiuria or urinary incontinence.

What is Cas 1451-82-7 used for?
It is employed as an important intermediate for raw material for organic synthesis, agrochemical, pharmaceutical, and dyestuff fields. Also used as an intermediate for 4-methylmethcathinone.
2-bromo-4-methylpropiophenone is employed as an important intermediate for raw material for organic synthesis, agrochemical, pharmaceutical, and dyestuff fields.
Specifically, this chemical can act as the intermediate in the synthesis of 4-methyl methcathinone HCl (mephedrone HCl or 4-MMC HCl), which is used as the sample for the development and validation of a presumptive color spot test method for the detection of piperazine analogs in seized illicit materials.

4-MMC HCl is an illegal drug, which takes a lot of side effects, such as dilated pupils, poor concentration, short-term memory loss, illusion, delusions and erratic behavior, so it is necessary to develop the related reagents and ketone products to detect its abuse
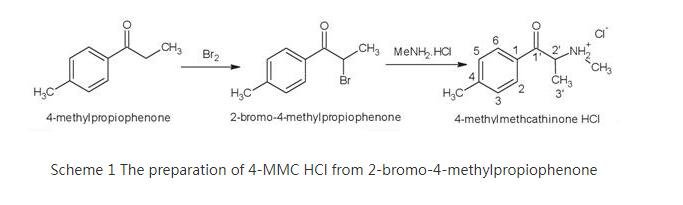
4-MMC HCl was synthesized in two stages (Scheme 1).
The first α-bromination step involved reacting 4-methylpropiophenone with excess bromine (to form 2-bromo-4-methylpropiophenone) in the presence of glacial acetic acid at 25 °C for 1 hour. The reaction solution was poured into ice-cold water and the 2-bromo-4-methylpropiophenone was extracted with dichloromethane and concentrated under vacuum to form yellow, fluffy crystals.
The final methamination step involved combining equal molar NaOH and methylamine hydrochloride solutions. This solution was then added dropwise over 1 hour to a stirred solution of 2-bromo-4-methylpropiophenone in toluene, and the mixture was allowed to stir for 32 hours at 25 °C and poured into ice-cold water.
The toluene layer was separated and acidified with dilute HCl solution, then the acidic extracts were washed with toluene before evaporating the aqueous layer to dryness to afford the crude 4-MMC HCl product as mottled light brown/brown colored, flaky crystals.
Finally, the fine, white powder was collected following recrystallization from isopropanol. The final product 4-MMC HCl was analyzed by using various chromatographic, spectroscopic, and mass spectrometry platforms and X-ray crystal structure analysis. These analyses are helpful to set up a rapid qualitative analysis of 4-MMC HCl.